
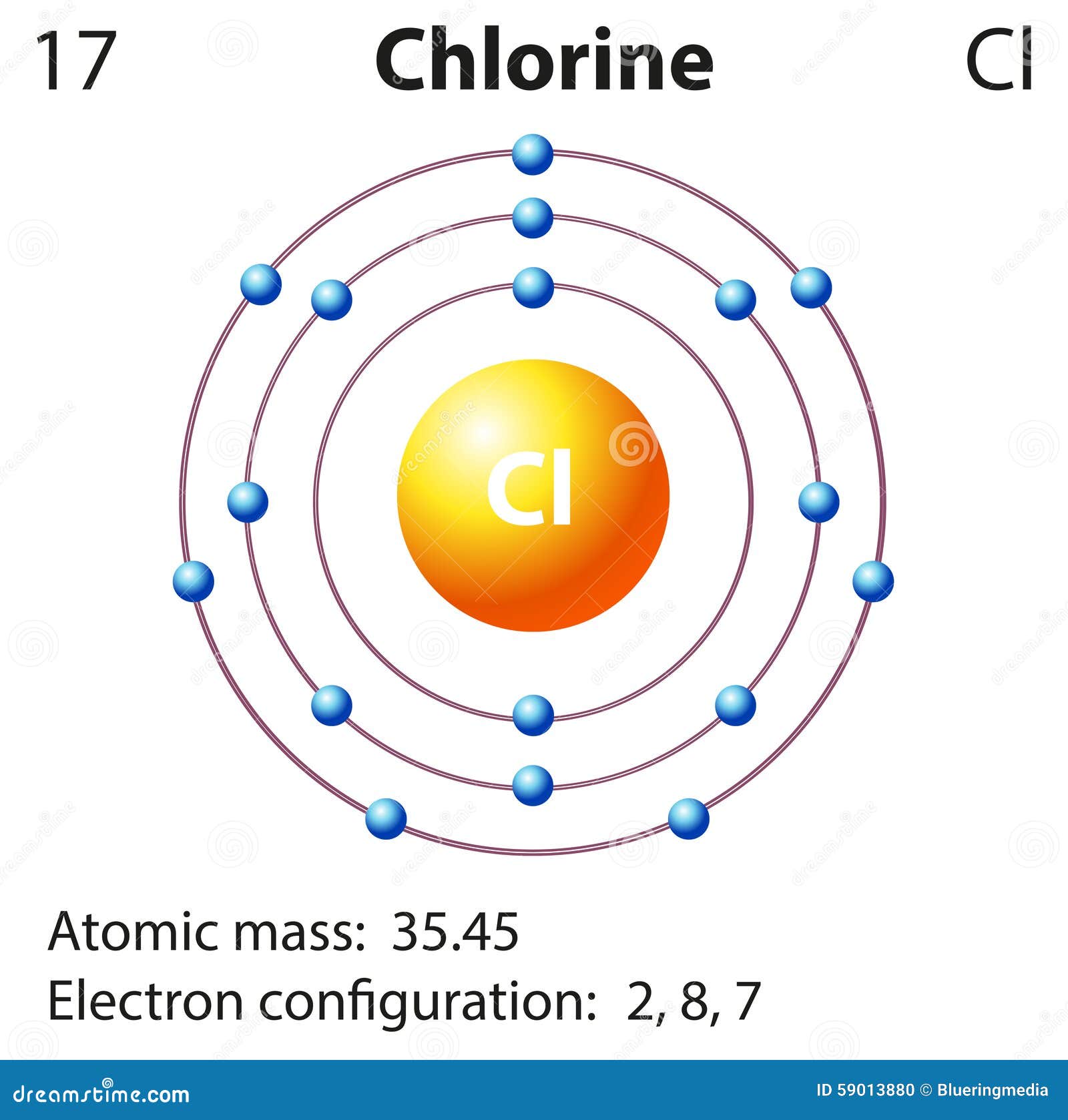
The mole amounts above represent the number of atoms of each element in the substance's molecular formula. Divide all the mole values by this amount:įinally, round each mole amount to the nearest integer to get the final mole ratios: The element with the fewest moles is Chlorine, with only 109.43450260246 moles. Use the mass to find the number of moles by dividing the mass by the molar mass (from step 1) of each element:įind the element with the fewest moles. For calculating numerical and in many reactions, we use the atomic mass of chlorine is 35.5u because when we calculate the average atomic mass the value comes. Magnesium chloride is made out of one magnesium atom and two chlorine atoms. Since the entered percentages do not add up to 100% (current sum is 91.5%), normalize them to sum to 100% by dividing each percentage by the sum of the entered percentages.Īssume you have 100g of substance to find the mass of each element by multiplying 100g by the percentage: The Ar of magnesium is 24 and the Ar of chlorine is 35.5. The the molar mass of an element is equal to the atomic weight. (a) The relative atomic masses of any element is the weighted average of the relative atomic masses of its natural isotopes.

Using a periodic table, find the molar mass of each element.
CHLORINE 35.5 HOW TO
How to find the empirical formula for 35.5% Chlorine, 56% Ironįinding the empirical formula of s substance that is 35.5% Chlorine (Cl), 56% Iron (Fe) requires just a few easy steps.
You can also ask for help in our chat or forums. Read our article on how to determine empirical and molecular formulas. 4.5 grams of metallic chloride contains 35.5 gram of chlorine.the equivalent mass of metal is a) 19.5 b)35.5 c)39 d)78 Dear Amit It should be 74.5 gm.

How To Determine Empirical/Molecular Formulas


 0 kommentar(er)
0 kommentar(er)
